A Characteristic Used to Describe a Substance Is Called
In order to observe the chemical properties of a substance the substance must be changed into a different substance. 2 Intensive depends on the.
Describing And Classifying Matter Let S Talk Science
Are characteristics that describe matter.

. A characteristic property is a chemical or physical property that helps identify and classify substances. Minerals Are Inorganic Minerals dont belong to any class of organic compounds which include substances such as carbohydrates proteins and fats made by living things. Glass plastics rubber starch Teflon polyvinyl chloride PVC polyurethane etc.
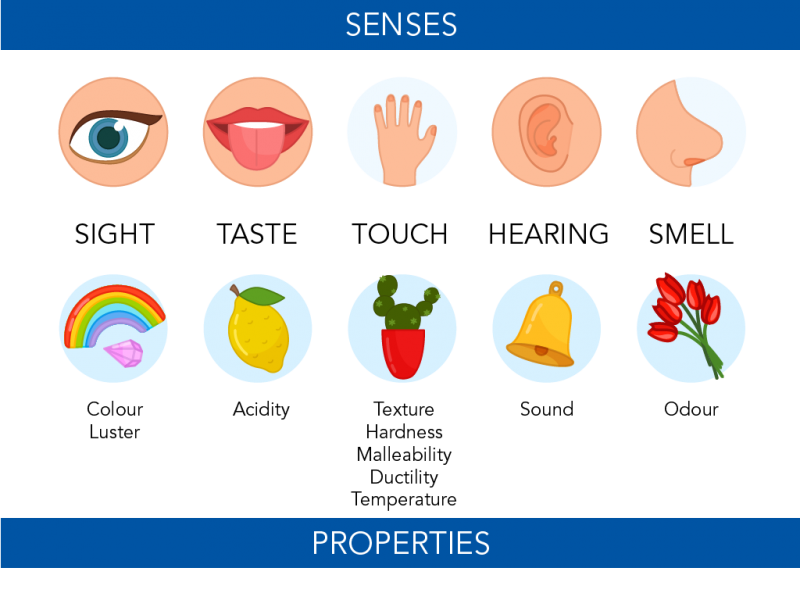
Specific gravity is the ratio between the mass weight of a mineral and the mass weight of an equal volume of water. We detect and observe the physical properties of matter using our five senses. Extensive and intensive properties.
Thus conversely if the property of a substance changes as the sample size changes that property is not a characteristic property. The properties that chemists use to describe matter fall into two general categories. A minerals specific gravity SG can be determined by dividing its weight in air by the weight of an equal volume of water.
A property is a characteristic that can be used to describe and identify matter. The characteristics of the substances determine the boiling and the melting point of the mixture. A charactristic that describes matter such as size shape and color.
There is no change in the energy during the formation of the mixture. The distinguishing characteristics or qualities that are used to describe a substance such as metal are known as its physical properties. A characteristic of a substance that can be observed and measured without changing the identity of the substance is called a physical property.
What is the characteristic of a substance that determines how it will react with other substances. A characteristic property of matter is a chemical or physical property that can help to identify or distinguish one substance from another. The characteristics that enable us to distinguish one substance from another are called properties.
This activity should take you about five to ten minutes. Extensive properties such as size and weight change when the amount of substance changes while intensive properties such as density and luster are independent of the amount. The arrangement of constituent particles atoms molecules or ions in such a solid has only short-range order.
Substances called mineraloids may look like minerals but are not because they dont satisfy all the requirements for being so. All the state of matter that is solid liquid or gases can combine to form a mixture. Physical properties are those that can be observed with the senses without changing the identity of the substance.
What makes living things different from non-living things. Chemical properties describe how a substance can be changed into a new substance. An ideal fuel must have an easily attainable ignition.
A substance whose constituent particles do not possess an orderly arrangement is called an amorphous solid. A numerical measure computed to describe a characteristic of a. An attribube that can be used to help identify ab substance.
A charactristic that describes matter such as size shape and color. Physical properties are divided into two groups. For instance quartz with a density of 265 is 265 times as heavy as the same volume of water.
All substances have characteristic physical and chemical properties. An individual living thingsuch as an animal or a plant is called an organismThe term living organism is usually used to describe something which displays all the characteristics of living things. They include characteristics such as size shape color and mass.
Charateristic of a substance that describes its ability to change into different substances. Physical properties - a characteristic that can be observed or measured without changing the identity or composition of the substance Physical properties used to describe matter can be classified as. Familiar examples of physical properties include density color hardness melting and boiling points and electrical conductivity.
A physical property is a characteristic of matter that is not associated with a change in its chemical composition. The characteristic properties of a substance are always the same whether the sample being observed is large or small. Taste and smell are also contact senses in which contact with a.
Conductivity is a physical characteristic representing a substances ability to conduct electricity. Sight hearing touch taste and smell. The temperature at which a substance catches fire is known as its ignition temperature.
1 Extensive depends on the. Chemical properties of matter include flammability reactivity with acids and corrosion. Those physical properties which describe the behavior of a metal when it is subjected to particular types of.
They include characteristics such as size shape color and mass. A charcateristic property is not affected by the amout or shape of a substance. Are characteristics that describe matter.
Some physical properties of a substance are density solubility melting point color and mass. Look at Figure 1. Mass volume length.
The properties that chemists use to describe matter fall into two general categories. Some Properties of Ideal Fuel are. This characteristic is called reactivity.
A substance that is formed from one type of atoms with a characteristic set of physical and chemical properties is called an element. Physical and chemical properties such as color density boiling point. Non-renewable resources must be used judiciously before their use all other alternative sources of energy must be taken into consideration.
Of matter in the sample - eg.

Pin By Jakob Fiudo On Stuff Amish Broom Brooms Intense

Fonts Used Acta Display Calibri Typewolf Typography Inspiration Typography Inspiration Typography Silicone Food Covers

1 4 Classification And Properties Of Matter Chemistry Libretexts
Comments
Post a Comment